FlowXl facility
FlowXl is a new research facility which aims to provide a state of the art laboratory for studying crystallisation processes, in-situ, through combined Raman spectroscopy and powder X-ray diffraction for probing solution-state molecular detail through to phase identification and quantification of materials in flow systems.
The FlowXl facility (Figure 1 left) is equipped with a rotating anode X-ray source used in transmission geometry with a 2D hybrid photon counting detector, and a Raman microscope with fibre-optic probe for in-situ measurements. The facility also has multiple crystallisation platforms which aims to provide users with a base set of equipment enabling the study of batch and continuous processes together with a number of microfluidic segregated flow platforms for studying crystallisation in controlled environments. The facility has a 0.5L process jacketed crystallisation vessel for traditional batch experiments, continuous crystallisation fReactor setup for studying anti-solvent and additive crystallisation, an acoustic droplet levitator for studying crystallisation within droplets under evaporation and a variable humidity cell for monitoring the impact of environmental conditions on the stability and transformation pathways of crystalline and amorphous materials.
Recent commissioning experiments have followed the crystallisation pathway of levitated droplets of sodium sulphate identifying a precursor liquid like phases prior to crystallisation and measurement of the phase transformation kinetics from a hydrated phase to its anhydrate structure (Figure 1). Overall, this initial case study highlights the power of FlowXl in terms of providing an accessible laboratory based facility for the crystallisation community to probe dynamic crystallisation pathways in real time.
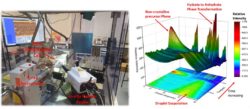
Figure 1. (left) FlowXl laboratory showing the X-ray instrument and Raman probe inside the enclosure with a flow crystallisation cell mounted on the goniometer, (right) X-ray diffraction data following the evaporation of an aqueous Na2SO4 droplet; capturing the evaporation, followed by a non-crystalline precursor phase, crystallisation of the decahydrate metastable phase before recrystallization phase transformation to the stable anhydrous phase
